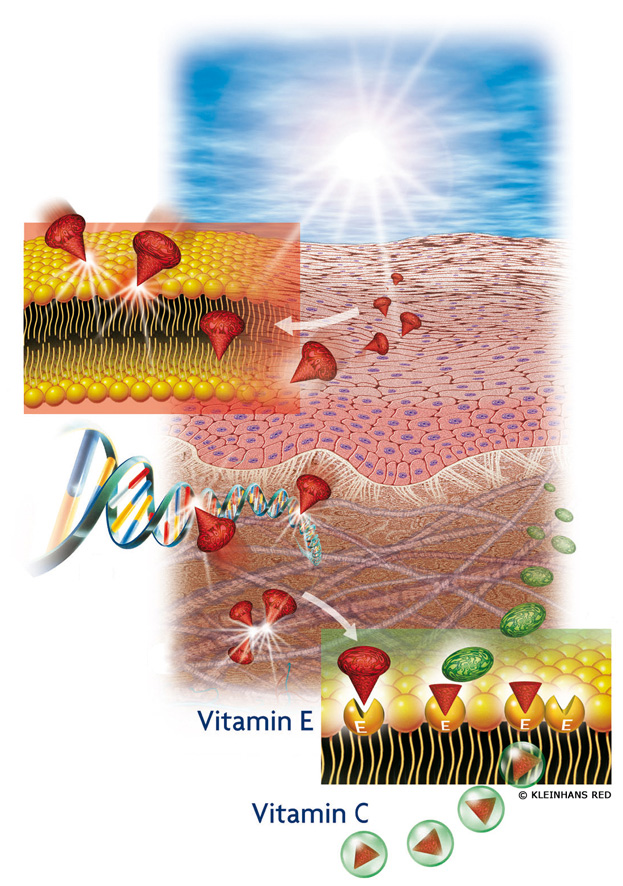
Vitamin E and vitamin C interact with each other in the skin to protect it from free radical attack

The human skin is an organ which is constantly embattled by the environment. Chemicals and environmental influences attack its health and appearance.
A well-known phenomenon is skin aging due to sun exposure (photoaging): ultraviolet (UV) and infrared (IR) radiation accelerates the aging process. An exposure to ultraviolet rays leads to the formation of free radicals. Antioxidative substances in the skin such as vitamin E and vitamin C are destroyed after exposure to ultraviolet rays.
Free radicals are highly reactive molecules. Their damaging potential may be counteracted by antioxidative substances in the skin such as the lipid-soluble vitamin E and the water-soluble vitamin C: If these antioxidants are topically applied or if they are replenished with food or dietary intake, they implement a protective effect by destroying the vitamins first, before destroying DNA, proteins and other important molecules.
With increasing UV exposure the antioxidative defense is compromised and an increasing amount of free radical species escapes destruction through the antioxidative defense system. These free radicals may then cause various types of cell damage, including lipid peroxidation as well as a modification of protein and DNA. Such damage may lead to premature aging and more serious consequences, such as initiation of skin cancer.
The Figure shows how vitamin E traps free radicals in the skin cells, while vitamin C supports this function. The free radical molecules (symbolized in red) damage the skin in the cell membrane (see (1) enlarged area), DNA in the cell nucleus (2) as well as in the collagen fibres (3), which are responsible for the tightness of the skin. Vitamin E may be stored in the cell membrane and may take away -- figuratively spoken the "point" of the molecule -- thus quenching its damaging potential. The free radical molecules (symbolized in red) thus lose their damaging effect (symbolized in green). Vitamin C then has the task to remove the "point" from the vitamin E and to release it again. Thus, vitamin C and vitamin E interact with each other and constitute an interplay between water-soluble and lipid-soluble antioxidants in protection against free radical damage.
Reference
- L. Packer, Ultraviolet irradation, free radicals and skin aging, Skin Care Forum No. 3 (1992)